Physicochemical processes leading to corrosion-passivation of metals and alloys: Understanding of the physico-electrochemical reactions at the metal|electrolyte interfacial system during the metal generalized and pitting corrosion on the basis of a point defect model and the nonlinear dynamical response of the system including periodic and chaotic oscillations of the current or potential under potential or current control, respectively.
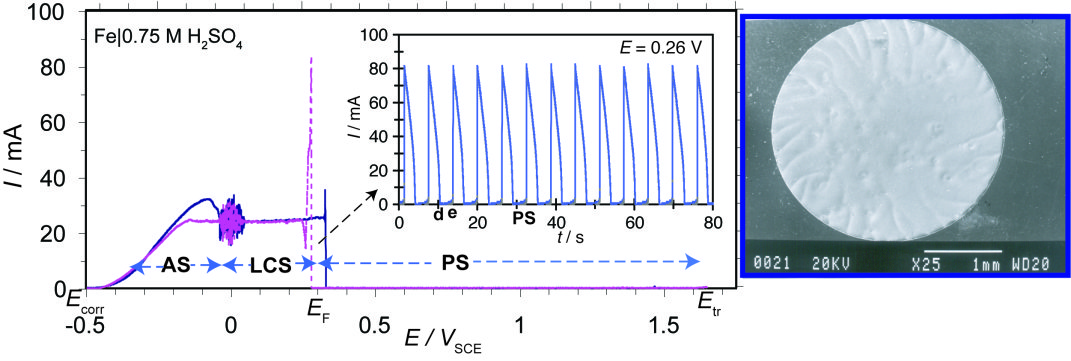
Current-potential (I-E) polarization curve of the Fe|0.75 M H2 SO4 system under potentiodynamic conditions (dE/dt=2 mV s-1) and current oscillations across the passive-active transition at potentials lower than the Flade potential E F. The current oscillations are monoperiodic, characteristic of the generalized corrosion, as the SEM micrograph of the Fe-disc electrode shows.
Potential control 2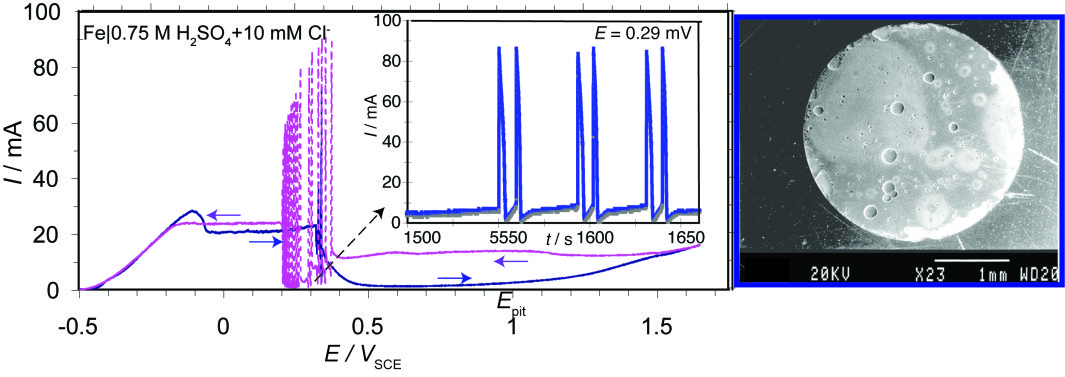
Adding a small amount of chlorides results in a wide oscillatory potential region across the passive-active transition extended towards the passive state and in complex periodic (or aperiodic) current oscillations, characteristic of the localized (pitting) corrosion induced by chlorides, as the SEM micrograph of the Fe-disc electrode shows.
Current control 1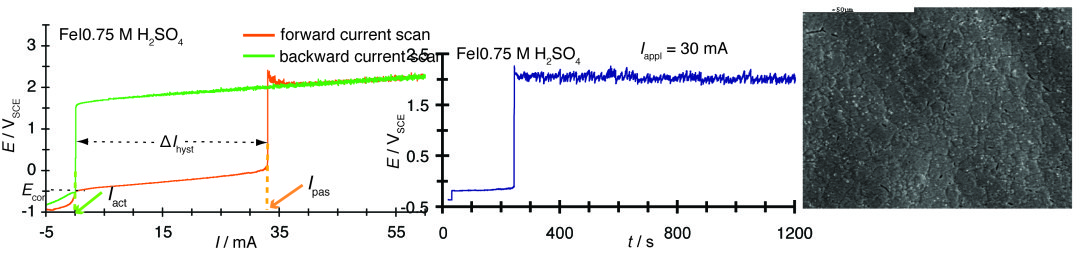
Potential-current, E=f(I), polarization curve of the Fe|0.75 M H2 SO4 system under potentiodynamic conditions (dI/dt=50 μA s-1) exhibiting bistability due to the hysteresis (Δ/hyst) between the transition to the passive state (Ipass) and the reverse transition to the active state (Iact), without potential oscillations under either under galvanodynamic (in the E=f(I) curve) or galvanostatic (in the E=f(t) curve, where fluctuations are due to the oxygen evolution reaction) contidtions.
Current control 2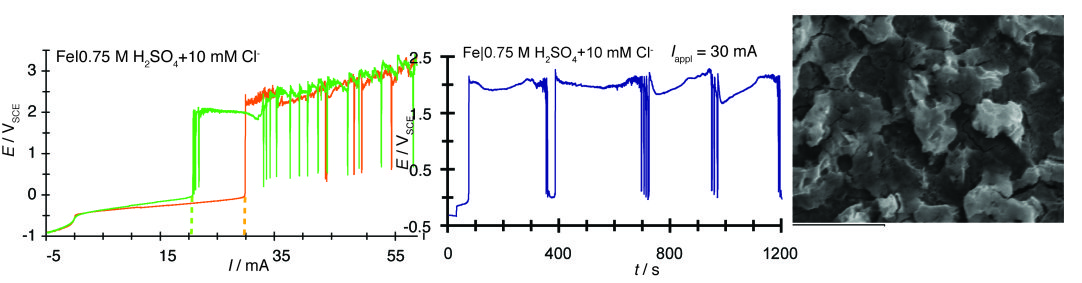
Adding a small amount of chlorides results in potential oscillations under both galvanodynamic (in the E=f(I) curve) and galvanostatic (in the E=f(t) curve) conditions due to the pitting corrosion induced by chlorides and the transformation of the oxide passive layer to an unstable mixed oxo-hydroxo-chloro layer, the morphology of which is shown in the SEM image taken during potential oscillations.
Phenomena of spatiotemporal self-organization of oscillatory chemical reactions
Briggs-Rausher reaction
A Modified Version of the Briggs-Rauscher ReactionThe Briggs-Rauscher (B-R) reaction is perhaps the most visually impressive chemical oscillator consisting of three colorless solutions (KIO3 + H2SO4, malonic acid + Mn(II)ions + starch and H2O2), which combined in a beaker and stirred on a magnet stirrer constitute an oscillating solution with rhythmic color changes: the colorless mixture becomes amber, blue and then colorless again. The B-R reaction is a hybrid of two other reactions: the Bray-Liebhafsky (B-L) and the Belousov-Zhabotinsky (B-Z). Briggs and Rauscher combined the H2O2 and iodate of the B-L reaction with the malonic acid and Mn(II)ions of the B-Z reaction. In the Briggs-Rauscher reaction, the evolution of O2 and CO2 gases and the concentrations of iodine and iodide ions oscillate.
Belousov-Zhabotinsky reaction
Homogeneous Belousov-ZhabotinskyBelousov-Zhabotinsky (B-Z) reaction is the most "famous" and well-studied chemical oscillator or "chemical clock" during which the color of the reaction mixture shows temporal periodic changes between colorless-yellow or red-blue and is considered today as the prototype system for nonlinear chemical dynamics. The original mixture consisted of bromate, citric acid and Ce(III) ions (as a catalyst). Zhabotinsky, discovered that oscillations still occurred if certain other organic compounds, such as malonic acid, were substituted for citric acid, also if other one electron transfer catalysts, such as Mn(II)ions, were substituted for Ce(III) ions and that ferroin[Fe(II)/Fe(III)] could act on its own as a catalyst. In the B-Z reaction the concentration of Br-, and the concentration ratio of Ce(II) and Ce(III) ions or other catalyst oscillate.
Non Homogeneous Belousov-Zhabotinsky
In an unstirred thin layer (i.e. in a petri dish), the ferroin-catalyzed B-Z system generates spontaneously spatial patterns or "chemical waves" consisting of spirals or concentric rings. As seen in video, blue rings or spirals are generated in an initially homogeneous red solution. This self-organization phenomenon of the B-Z served as an analogue of understanding similar spatial patterns seen in biological systems such as aggregating patterns of the amoebae cells Dictyostelium discoideum. The cellular slime mold D. discoideum is considered as a suitable system to study cellular communication, biological oscillations and differentiation involved during its characteristic morphogenesis.
Anticorrosion protection of metals and alloys by intrinsically conducting polymers and micro-nanocomposite coatings based on micro- nano-conducting polymers.
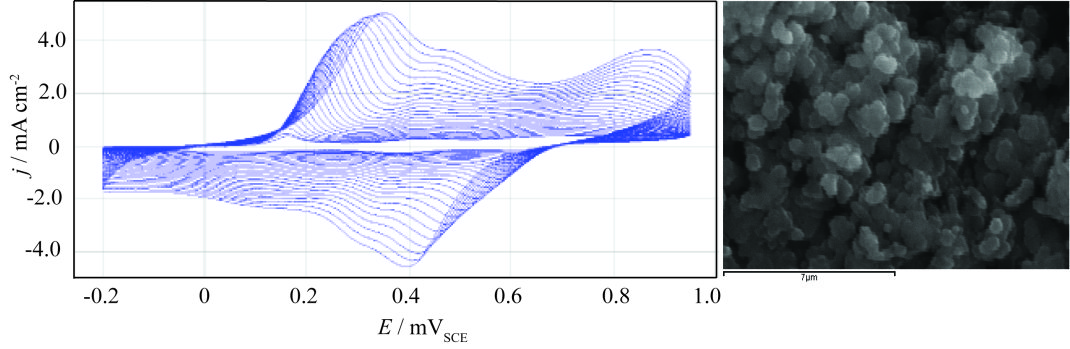
Growth of polyaniline (PAn) by cyclic voltammetry (dE/dt=20 mV s-1) on passive stainless steel AISI304 from 0.1 M H2 SO4+0.05 MAn solution and the morphology of the PAn film.
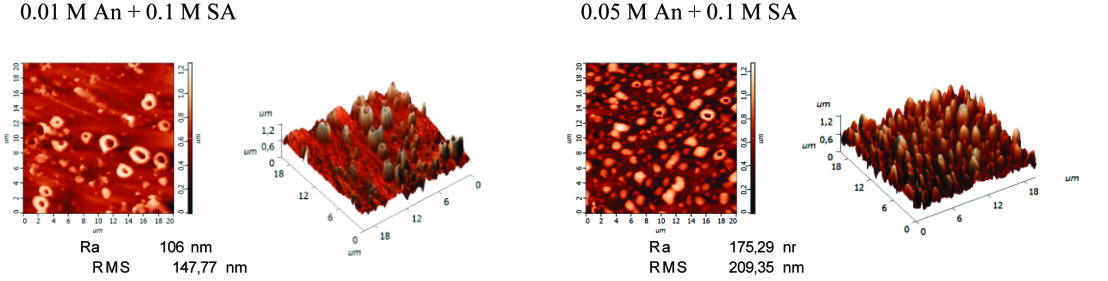
Electrochemical copolymerization of aniline (0.05 M) and 4-aminobenzosulfonic acid (0.1 M) on stainless steel (AISI304)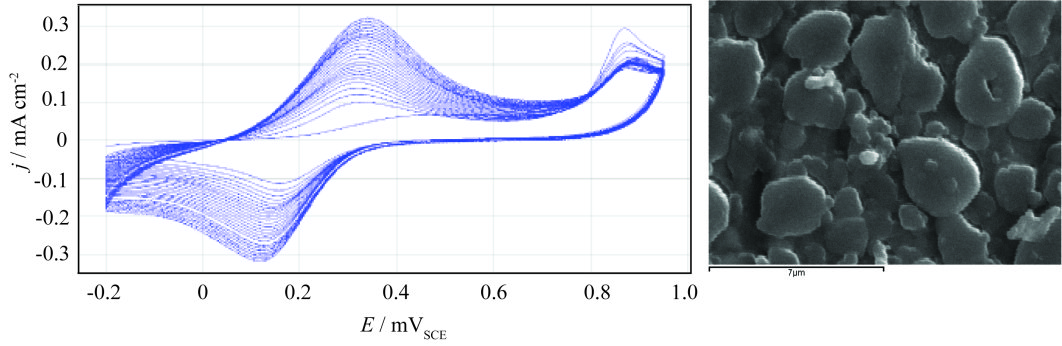
Growth of poly(aniline-co-4-aminobenzosulfonic acid) P(An-co-4-ABSA) by cyclic voltammetry (dE/dt=20 mV s-1) on passive stainless steel AISI304 from 0.05 M An+0.1 M 4-ABSA solution, without the presence of a supporting electrolyte, and the morphology of the copolymer film.
Formation of hollow microspheres